On Monday, at the sensational conference, the news of the discovery on Mars of water in its liquid form, rushing down hillsides, "exploded". This statement made the whole world turn to the Red Planet ... once again. At the conference, scientists provided evidence confirming the existence of water (in liquid form) on the surface of a drained planet. It turns the seemingly desolate world into a real oasis. In any case, that was the meaning of the newspaper headlines.
Of course, water was found on Mars before. We know that water in a bound state is in the Martian regolith, that it is enclosed in the ice caps of the Martian mountains and in the ice reserves under the surface. We also know that rivers once flowed on Mars, and lakes and whole seas were filled with water. In recent years, an armada of terrestrial orbital stations, landing modules and rovers has fundamentally revolutionized our understanding of this neighbor of the earth. But this time the Martian Orbital Probe made complex measurements of what turned out to be seasonal flows of liquid water on the surface of modern Mars. Moreover, this phenomenon occurs on a global scale.
This may be the most significant discovery associated with Martian water, but at the same time, the most frightening. It may make us regret that water on Mars exists at all.
Follow the Waters
Let's go back to Earth for a minute. Today, the Earth is the only planet in the Universe, the existence of life on which we know for sure. It is in a privileged position relative to the sun. This area is called the "Habitability Zone". It is characterized by the fact that the distance to the nearest star allows water to be in a liquid state. And that is the key to the "Earth Version" (™) of life. All forms of this life, as far as we know, in one way or another use liquid water in their metabolism. Where there is water, there is life. But does this logic work in other worlds of the Universe? Does it act on other planets or the moons of our solar system? Does it act on Mars?
Until recently, the only observed forms of the existence of water on Mars were its gaseous or solid state. The atmosphere of the planet is too cold and too rarefied to maintain the existence of water in a liquid state (at least on the surface). This was not always the case. In the early stages of the evolution of the solar system, Mars was a blue planet. Even before this was the Earth. Once it had a dense atmosphere. But in antiquity with Mars, something happened, and this something "turned off" the internal dynamo that generated the global magnetic field. Having lost the magnetosphere, Mars put the atmosphere of its planet under the destructive influence of the solar wind. And the planet has undergone a deep draining freeze.
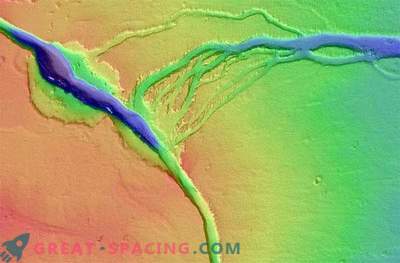
Water on the surface of Mars is frozen or sublimated (turned from steam into ice, bypassing the liquid phase). Part of the water vapor dispersed into space. Huge reserves of water, most likely, turned out to be enclosed under the surface in the form of ice, in areas of permafrost, or lingered in the ice caps of the mountains. They became frozen artifacts of the once full moisture of the world. But in 2011, experts, analyzing the data of the Martian Orbital Probe, reported on the detection of strange seasonal dark bands that appear on the sun-heated Martian slopes in the southern hemisphere. And then, using indirect data, the scientists for the first time tied up "repeating lines on the slopes" (PLS, or RSL) with streams of liquid water on the surface of Mars.
More recently (in one week with the presentation of the film "The Martian" with Matt Damon), this assumption was confirmed. Using a spectrometer installed on the Orbital Probe, spectral lines of hydrated salt were detected in the dark RSL lines. This finding was a confirmation that the dark lines are indeed streams of water that are in a liquid state, but only supersaturated with salts. That is, we have perchlorate brine. This is amazing news. It confirms that today there is liquid water on the surface of Mars. But it is quite possible that this should not please future researchers. In fact, it can pose a mortal danger to them.
Martian antifreeze
Behind the hype raised in the press, it’s hard to imagine that the presence of water on Mars can look like bad news. But for the hypothetical astronauts of the future, water is not necessarily salvation. In any case, they are unlikely to drink it from the springs.
In the 1970s, NASA sent two automatic Viking stations for Mars exploration. The purpose of the landing modules was the search for life. These first robots were carried out experiments on the study of local soil. One of the experiments, which was developed to determine the signs of metabolism of Martian bacteria, gave positive results. After the nutrient substances were added to the Martian regolith samples, a gas emission was recorded. Such a reaction can be expected from microscopic life forms, which are beginning to develop dramatically, like an effervescent tablet thrown into water. However, at that time, the experiment was recognized as an error, since no trace of organic compounds was found later on in the same sample.
So, the Viking program could not unambiguously determine whether there is life on Mars. Therefore, funding for further experiments in this direction has become more complicated. But in connection with the new discoveries, it is quite possible that the following missions will again have to look for a “habitable environment” in the past or present of the planet.
In 2008, the descent vehicle Phoenix, descended in the Arctic region of Mars, made a new breakthrough in research. Perchlorates were found on the surface of the planet. These are extremely toxic chemicals that are used in various industrial processes on Earth. In particular, they can be oxidizing agents in rocket fuel. Interestingly, in this case, they can be compared with salt, which city services are scattered on the sidewalks in cold weather to lower the freezing point of water. In cities, this method is often used to prevent road icing. Perchlorates are very active compounds, they reduce the freezing point of water to the level corresponding to the cold Martian climate. That is, they form antifreeze of natural origin. Phoenix Station complemented the discovery of perchlorates with impressive images of what could be drops of brine rich in perchlorate on its landing module. Much later, the discovery of the Phoenix was confirmed by the Curiosity Martian rover, who conducted research in the equatorial region (inside the Gail crater), also abundant in perchlorates.
Dark (and light) sides of perchlorates
Since perchlorates are powerful oxidizing agents, they can destroy any organic compound when heated. Now we know that the Red Planet is covered with both perchlorates and organic compounds (which was also confirmed by Curiosity). Maybe we should review the results of the old Viking experiment? The main reason that Viking could not confirm the existence of life on Mars was the absence of organic compounds in the samples. But in 1970 we knew nothing about perchlorates. And it is quite possible that during the experiment they simply sterilized the sample. This is an interesting assumption.
Perchlorates are extremely toxic to humans. Even in small doses, they can cause very big troubles (cause thyroid disease). They are also considered a strong carcinogen. But Mars is rich in perchlorates. It is possible that they will become the main danger for the future exploration of Mars, exceeding even the radiation one. Their decontamination will be the main task. Mars is for the most part a very dry world and very dusty. It is known that this dust for the most part consists of hazardous substances. It is unlikely that future astronauts will want to breathe such air if the nearest hospital has to travel many millions of miles.
Of course, this does not negate the prospect of further exploration of Mars, but complicates the task a little. In fact, we are lucky that research robots discovered the toxic component in advance. Now we can better prepare for protection. Even on Earth, there are microorganisms that use perchlorates for energy. And this fact may be a sufficient condition in order to send an expedition to Mars, despite the danger. As scientists emphasized at the last press conference, we are unlikely to be able to send robots to confirm this discovery. The slopes on which this phenomenon was discovered are very steep, and the landing of any automatic vehicle in a given area is almost impossible (at least with the present level of technology development). Astrobiologists are currently considering these areas as a priority for finding life on Mars. Therefore, another problem may be pollution, terrestrial microorganisms can sneak into this area on automatic vehicles, and the results of research will be false. Earth microorganisms can even develop and litter the entire region. Of course, it is difficult to estimate the likelihood that some terrestrial bacteria will feel at home in poisonous brines of Martian water, but it will not be correct if the first organic life found on Mars is brought from Earth.
The study of these slopes is likely to be one of the priority tasks of the manned Martian expedition, which NASA plans to launch in the late 2020s or early 2030s.
And although the source of the "Repeated Slope Lines" is still unknown, their potential, as the birthplace of life, is seen very clearly. So let's try to determine once and for all whether there is life on Mars. And we will develop technologies that allow us to live on this planet. Perhaps these waters are poisoned. But still, you need to follow the waters on the Martian slopes.